Fabulous Tips About How Do Cathode Rays Move From Negative To Positive
Unveiling the Cathode Ray Mystery
1. What Exactly Are Cathode Rays, Anyway?
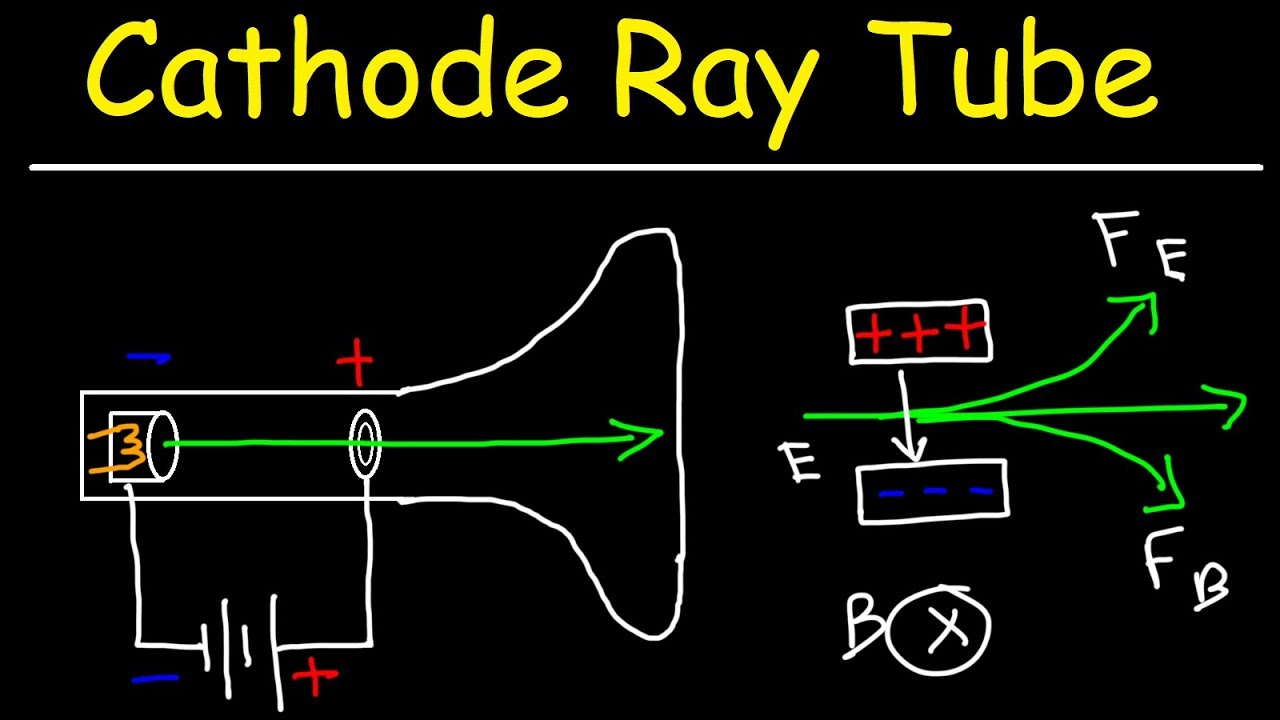
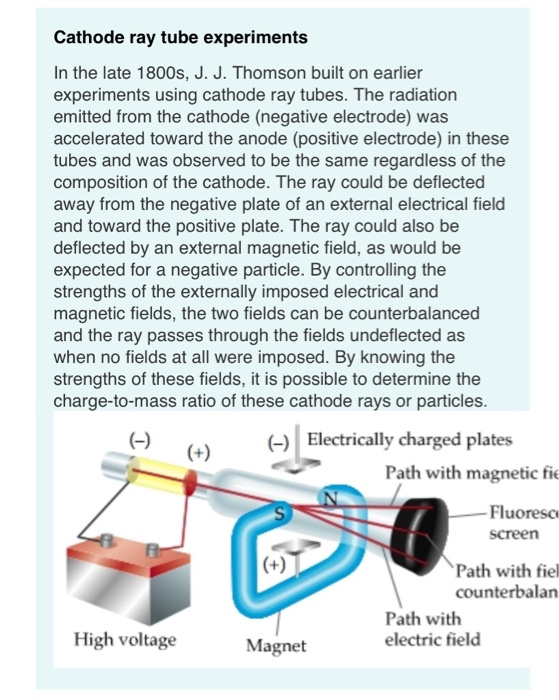
Okay, let's dive into the fascinating world of cathode rays. Picture this: it's the late 1800s, and scientists are playing around with vacuum tubes (the ancestors of your TV screen!). They noticed that when a high voltage is applied across the tube, a mysterious "ray" shoots out from the negative electrode (the cathode) towards the positive electrode (the anode). These rays could make certain materials glow, and they were dubbed "cathode rays."
Now, what are these rays made of? Initially, there was quite the debate. Some thought they were waves, like light. Others suspected they were particles. It took some clever experiments to finally nail down the truth. Turns out, cathode rays are streams of electrons tiny, negatively charged particles. And that's the key to understanding their movement.
Think of it like this: you have a bunch of mini negatively charged "bullets" being fired from one end of the tube to the other. Its not magic, its simply the fundamental laws of physics at play! This discovery was a major breakthrough, paving the way for things like television, computer screens, and a whole lot of other cool tech we use every day.
So, to recap, cathode rays are simply streams of electrons emitted from the cathode. These aren't just random beams; they are guided and directed by the electrical setup within the tube. Understanding their nature is the first step to grasping why they move from negative to positive.
The Electric Field
2. How Electricity Dictates the Electron Dance
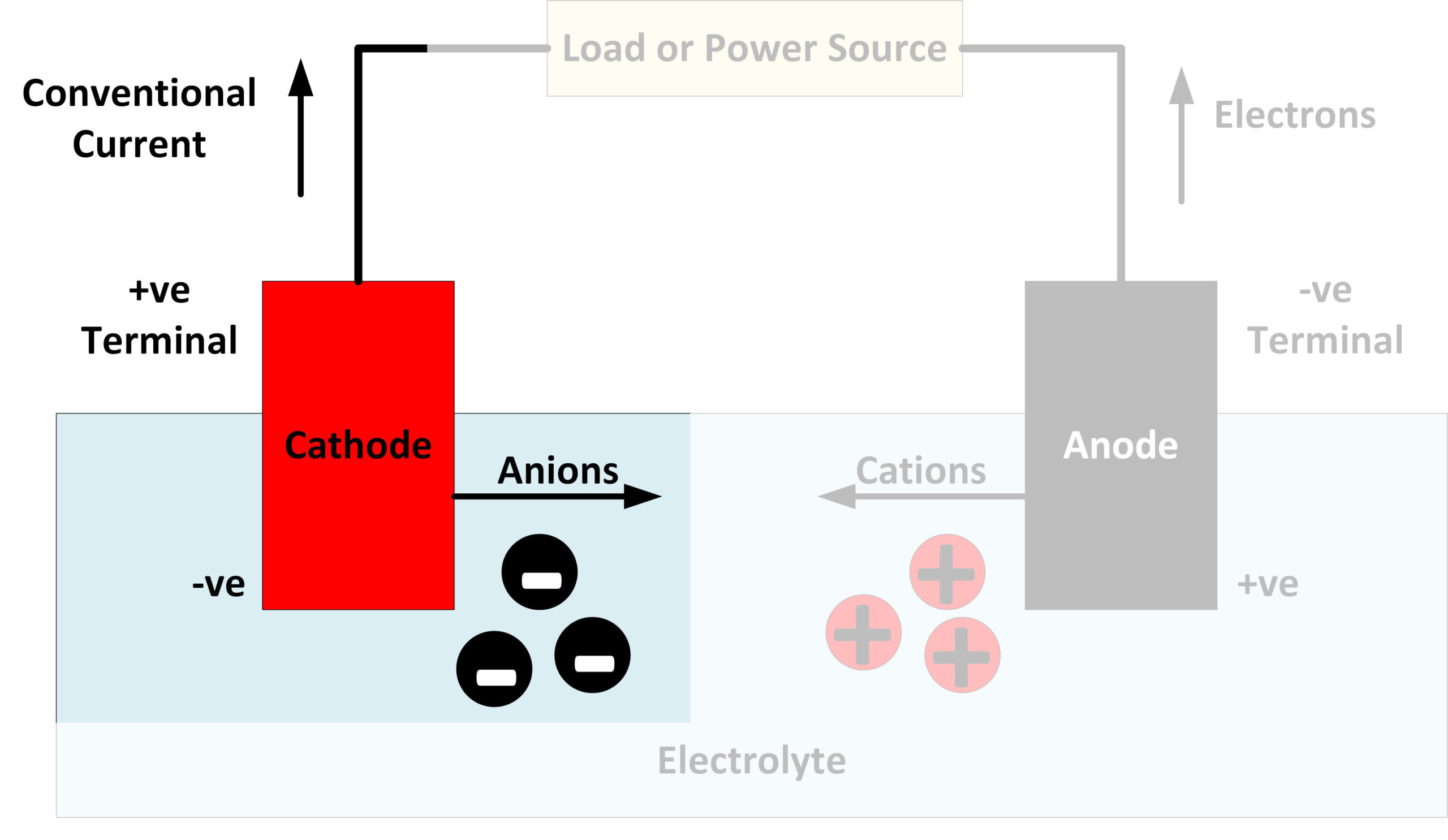
Alright, so we know cathode rays are made of electrons, and electrons are negatively charged. Now, let's talk about the electric field. Remember from science class that opposite charges attract, and like charges repel? That's the fundamental principle we need to understand here.
When a voltage is applied across the vacuum tube, it creates an electric field — essentially, a region of space where charged particles feel a force. The positive electrode (anode) becomes a region of positive charge, while the negative electrode (cathode) becomes a region of negative charge. Think of it like a hill, but instead of gravity pulling you down, it's electric force pulling the electrons towards the positive side.
Because electrons are negatively charged, they are attracted to the positive anode and repelled by the negative cathode. This attraction and repulsion create a force that propels the electrons across the tube. It's like a tiny tug-of-war, with the positive charge winning and pulling the electrons along for the ride.
So, the electric field acts like an invisible pathway, guiding the electrons from the cathode to the anode. Without the electric field, the electrons would simply sit there, doing nothing. It's the driving force behind the movement of cathode rays.
Cathode Led Positive Or Negative Bastatrain
The Vacuum's Vital Role
3. Why Empty Space is Crucial for Cathode Ray Movement
Now, you might be wondering why we need a vacuum tube in the first place. Why can't we just shoot electrons through the air? Well, imagine trying to run a race through a crowded room versus running on an open track. That's the difference the vacuum makes.
The vacuum removes almost all the air molecules from the tube. This is crucial because electrons are tiny and easily collide with air molecules. If there were a lot of air molecules in the way, the electrons would constantly bump into them, scattering them in all directions and losing energy. The cathode ray would quickly dissipate and never reach the anode.
By creating a vacuum, we provide a clear path for the electrons to travel. They can zip across the tube without significant interference, maintaining their energy and direction. It's like giving them a superhighway to reach their destination.
Think of it like this: the vacuum is the unsung hero of the cathode ray tube. It's the silent enabler that allows the electrons to travel freely and do their job. Without it, our TVs and other devices wouldn't work at all!

Atoms And Molecules. Ppt Download
Deflecting Cathode Rays
4. Magnets and More
Okay, so we know cathode rays travel in a straight line from negative to positive. But what if we want to control their path? Turns out, we can! By applying magnetic fields or electric fields, we can deflect the cathode rays, bending their trajectory and making them do all sorts of interesting things.
Imagine throwing a ball, and someone puts a giant magnet nearby. The ball's path would curve towards or away from the magnet, depending on the ball's material. Similarly, when a magnetic field is applied to a cathode ray, the electrons experience a force that causes them to move in a curved path. The direction of the curve depends on the direction of the magnetic field and the charge of the electrons.
We can also use electric fields to deflect cathode rays. By placing charged plates near the tube, we can create an electric field that pushes or pulls the electrons, changing their direction. This principle is used in oscilloscopes, where the deflection of the electron beam is used to visualize electrical signals.
This ability to deflect cathode rays is incredibly useful. It allows us to control the electron beam in a TV screen, painting images by scanning the beam across the screen. It also has applications in electron microscopes, where the deflection of electrons is used to create highly magnified images of tiny objects.
:max_bytes(150000):strip_icc()/how-to-define-anode-and-cathode-606452_FINAL-0fb3b21b79564acd9ef7c729a2bcd98e.png)
What Happens At The Cathode In Electrolysis
Cathode Rays
5. From Television to Modern Applications
You might be thinking, "Cathode rays? Isn't that old technology?" And while it's true that cathode ray tube (CRT) TVs are largely a thing of the past, the principles behind cathode rays are still very relevant today. The understanding of electron beams and their manipulation has led to numerous advancements in various fields.
For instance, electron microscopes, which use focused beams of electrons to image objects at a much higher resolution than optical microscopes, are indispensable tools in biology, materials science, and nanotechnology. These microscopes rely heavily on the principles of cathode ray technology, using electric and magnetic fields to control and focus the electron beam.
Even technologies that seem completely unrelated, like particle accelerators, owe a debt to the early work on cathode rays. Particle accelerators use electric and magnetic fields to accelerate and guide beams of charged particles to incredibly high speeds, allowing scientists to probe the fundamental building blocks of matter. The understanding of how to control electron beams was a crucial stepping stone in the development of these complex machines.
So, while you might not see cathode ray tubes in every home anymore, the knowledge gained from studying them continues to shape our world. They were a pivotal discovery in physics, leading to countless innovations that we benefit from every day.
